Recessive genetic conditions
There are several genetic conditions in Wagyu that have been recorded in Japan: Chediak-Higashi syndrome (CHS), Claudin 16 deficiency / Renal tubular dysplesia (CL16), Spherocytosis (B3), Factor XI deficiency (F11), Factor XIII deficiency (F13), Bovine Chondrodysplastic Dwarfism (BCD), Marfan Syndrome, Multiple Ocular Defects (MOD), Haemophilia A, Xanthinuria, IARS Disorder and Epilypsy.
Marfan syndrome has a dominant mode of inheritance. Most of these conditions are found in Black Wagyu. Conditions confined to Red Wagyu (Japanese Brown/Akaushi) are Haemophilia A and Bovine Chondrodysplastic Dwarfism.
Hereditary conditions in Wagyu outside Japan
Wagyu have been identified which are affected by, or are carriers of, the following known conditions in Australia:
CHS (Chediak Higashi Syndrome)
The bleeding disorder in cattle with a light coat colour is caused by an autosomal recessive mutation which is caused by the substitution of a nucleotide on the LYST gene. There is also depressed immunity and the first sign is usually excessive bleeding from the umbilical cord at birth.
Carriers that have been identified from the initial testing of foundation animals are Itomoritaka "002" (J2703, FB681 and IMJFAJ2703), Itoshigefuji TF147 (FB3681 and IMUFQTF147), Mazda (FB103 "C"and IMUFA0103 "FU"), Mitsuhikokura TF149 (FB3683 and IMUFNTF149), TF601 (FB5999 and IMUFZ5999) and TF603 (FB6188 and IMUFZ6188). Itomichi (PEDFA500 and FB500) has a 99% probability in GeneProb of being a carrier.
CL16 (Claudin 16 deficiency Type 1)
This condition in Black Wagyu is also called Renal Tubular Dysplasia and is a lethal autosomal recessive condition usually resulting in death by six years of age. Kidney failure and retarded growth are caused and can be associated with overgrown hooves and diarrhoea.
Type-1 mutation is caused by a 37kb deletion and the more recent Type-2 mutation is caused by a 56-kb deletion.
The frequency of the CL16 deficiency mutant gene in Japan is 0.1. Foundation animals Yasufuku Jr (FB5061 and PEDFS100), JVP Yasutanisakura 931 (FB2102 and IMUFN2102) and Kimifuku TF726 (FB7584 and IMUFB7584) are known to be carriers of CL16.
B3 (Spherocytosis)
Low birth weight is followed by laboured breathing from moderate anaemia with spherocytosis, retarded growth and acidosis. It is lethal and death usually occurs within ten days after birth. If survival goes beyond that, adulthood is often reached despite retarded growth. A CGA to TGA substitution and Arg to Stop mutation is the cause of dominantly inherited spherocytosis.
The frequency of Spherocytosis mutant gene in Japan is 0.04. Foundation bull, and AI approved sire, JVP Yasutanisakura 931 (FB2102 and IMUFN2102) is the only known carrier of B3 to have been confirmed in Australia. As a carrier of B3, CL16 and F11, there are very low numbers of his progeny - 124 progeny have been registered by AWA (Australia). Several have died and none of his registered progeny were born after recessive testing commenced in Australia. However some of these progeny have bred and their progeny show a probability of 25% to be carriers of B3 in GeneProb. JVP Yasutanisakura 931 has 92 progeny registered by AWA (USA) of which 9% were born in 2010 but none have been registered subsequently.
IARS (Isoleucyl‐tRNA synthetase) disorder
IARS disorder causes low birth weight after normal gestation, weakness and poor suckling. Symptoms in calves that are affected are anaemia, depression, weakness,  variable body temperature, difficulty nursing, growth retardation, inability to stand unassisted and increased susceptibility to infection. Inseminations are repeated between 61 and 140 days due to embryonic or foetal deaths. The term of the pregnancy is often slightly extended and this suggests that the growth of the foetus is retarded when they survive through to birth. A mortality rate of more than one half of homozygous recessive (affected) embryos was reported by Hirano et al., 2016.
variable body temperature, difficulty nursing, growth retardation, inability to stand unassisted and increased susceptibility to infection. Inseminations are repeated between 61 and 140 days due to embryonic or foetal deaths. The term of the pregnancy is often slightly extended and this suggests that the growth of the foetus is retarded when they survive through to birth. A mortality rate of more than one half of homozygous recessive (affected) embryos was reported by Hirano et al., 2016.
A random sample of 1,380 assays gave the mutant gene frequency (C frequency) of 0.070. In comparison, Australian Wagyu Association members have conducted approximately 90,000 genomics tests in the past three years. Analysis of the SNP on these genomics chips identified that the profiles of half of these contained the SNP that is used for determining IARS status. The mutation frequency is 0.089 and the incidence of carriers is 17.2%.
Table 1: The comparative results are shown in this table:
|
Total |
Free |
Carrier |
Affected |
Carrier |
C gene |
Japan |
1,380 |
1,186 |
194 |
0 |
14.1% |
0.070 |
Australia |
44,839 |
36,991 |
7,701 |
147 |
17.2% |
0.089 |
The typical 25:50:25 offspring ratio is expected when two heterozygotes (G/C) are joined. However, compared to the expected numbers, there were significantly fewer affected animals in this population (P<0.05), suggesting that more than half of the affected embryos died before birth. The frequency of re-insemination after more than 11,580 carrier over IARS carrier inseminations was significantly (P<0.001) higher between 61 and 140 days. The findings suggested that the homozygous IARS mutation not only causes calf death, but also embryonic or foetal death.
Table 2: Assay results from IARS carrier x IARS carrier, Japan:
|
Total |
Free |
Carrier |
Affected |
Carrier |
C gene |
Actual |
92 |
27 |
55 |
10 |
59.8% |
0.408 |
Expected |
92 |
23 |
46 |
23 |
50.0% |
0.500 |
Results from GeneProb are posted on the Australian Wagyu Association website and the following Foundation sires have been identified to have a 99% probability of being Carriers: Fukutsuru J068, Haruki II, Itozurudoi TF151, Kikutsurudoi TF146, Kitateruyasudoi "003" and JVP Yasutanisakura 931.
It is anticipated that the number of IARS carriers in most herds is between 15 and 20%. It could be higher if ancestors that have IARS have been used more heavily. In a herd with a frequency of IARS carriers of 17% - as indicated in Australia - in the first generation of random mating, 83.7% of the offspring will be Free, 15.6% will be Carriers and 0.7% will be Affected. More than half of the Affected progeny are expected to succumb to IARS.
However when an IARS carrier bull is used over IARS carrier cows, 25% of the progeny will be affected and so overall mortality rate in that herd is expected to be around 15%. By testing individuals that are known to descend from the original Founder carriers, the carriers in today’s herds can be identified and the mating of carriers can be prevented.The Red Wagyu/Akaushi founders are considered to be untested free of IARS, but some progeny from Free Red Wagyu/Akaushi have been tested to be IARS Carriers after joining with Black Wagyu IARS Carriers. Testing of progeny from a Carrier is recommended.
Probability results for IARS and the other known recessive conditions are presented in tables at the end of this page.
Subsequent to the identification that the IARS disorder (specifically the isoleucyl-tRNA synthetase c.235G>C mutation) as one of the causes of weak calf syndrome in Japan, calf deaths differing from those attributed to IARS disorder have been occurring. Research has commenced with bulls that do not carry the IARS mutation but nevertheless have high calf mortality rates. In one trial reported by Takashi Hirano in 2017, it is suggested that the incidence of calf death in calves sired by one bull was a hereditary disease exhibiting a dominant, not recessive, inheritance pattern. This work is ongoing.
More detail on IARS Disorder can be viewed by clicking here.
F11 (Factor XI deficiency)
Tests carried out in Australia and USA have identified the Factor XI deficiency in both Red and Black Wagyu. This implies that the F11 mutation in Wagyu occurred before the segregation into Black and Red breeds. Foundation animals that are carriers for F11 are: Hikari (FB2455 and IMUFN2455), Itohana 2 TF38 (FB2294 and IMUFN2294), Itoshigenami TF148 (FB3682 and IMUFQTF148), JVP Fukutsuru 068 (FB2101 and IMJFMJ068), Kimifuku TF726 (FB7584 and IMUFB7584), Kitateruyasu 003 (FB686 and INJFAJ2810), Shigemaru (FB2124 and IMUFN2124), TF601 (FB5999 and IMUFZ5999) and JVP Yasutanisakura 931 (FB2102 and IMUFN2102).
Factor XI deficiency was documented in Holsteins in 1969. Affected animals take a longer time for blood to coagulate after injury or there is bleeding that doesn't stop from the umbilical cord after birth. Blood has been observed in cow's milk and this led to the diagnosis of this condition in Europe. The blood coagulation effect was found to be severe in affected homozygotes and partially deficient in heterozygote carriers. Reports from 1995 suggested effects on reproductive performance with a 50% greater incidence of repeat breeding of affected animals. A 76-base insertion mutation to exon 12 of the F11 gene is the cause. A heterozygote cow was the first Holstein to be isolated with the condition in Japan.
Factor XI deficiency is encountered in Wagyu and Holstein. There are some similarities in the clinical symptoms in the breeds. The causative insertion mutations are on different locations of the F11 gene and these variations are considered to be responsible for the differences in severity between the breeds.
Factor XI deficiency in Wagyu has an autosomal recessive mode of inheritance and is caused by an insertion of 15 nucleotides in exon 9 of the F11 gene. Frequencies of the mutated gene were found to be 26.4% from a random sample in Japan. Even though the number of carriers that are detected in testing exceeds this proportion in Australia, most tests that have been carried out currently are confined to high risk animals that are idrntified through GeneProb. It is not considered by Wagyu International that the incidence in Australia is any higher than the initial 30% when the foundation animals were first tested.
A laboratory trial involving a few animals in Australia indicated that bleeding time in the laboratory was 65% slower in affected animals when compared to carriers. The owner of the affected animals advised that they did not show any symptoms.
In Australia there was renewed interest in F11 when AWA presented a talk on it but no new data was provided. There are many factors involved in fertility but it would be comforting to know if iF11 is Wagyu has any effect as it is present in 30% of the herd and is more prevalent in higher marbling lines.
A table has been prepared to show the recessive status of the Wagyu Founder sires:
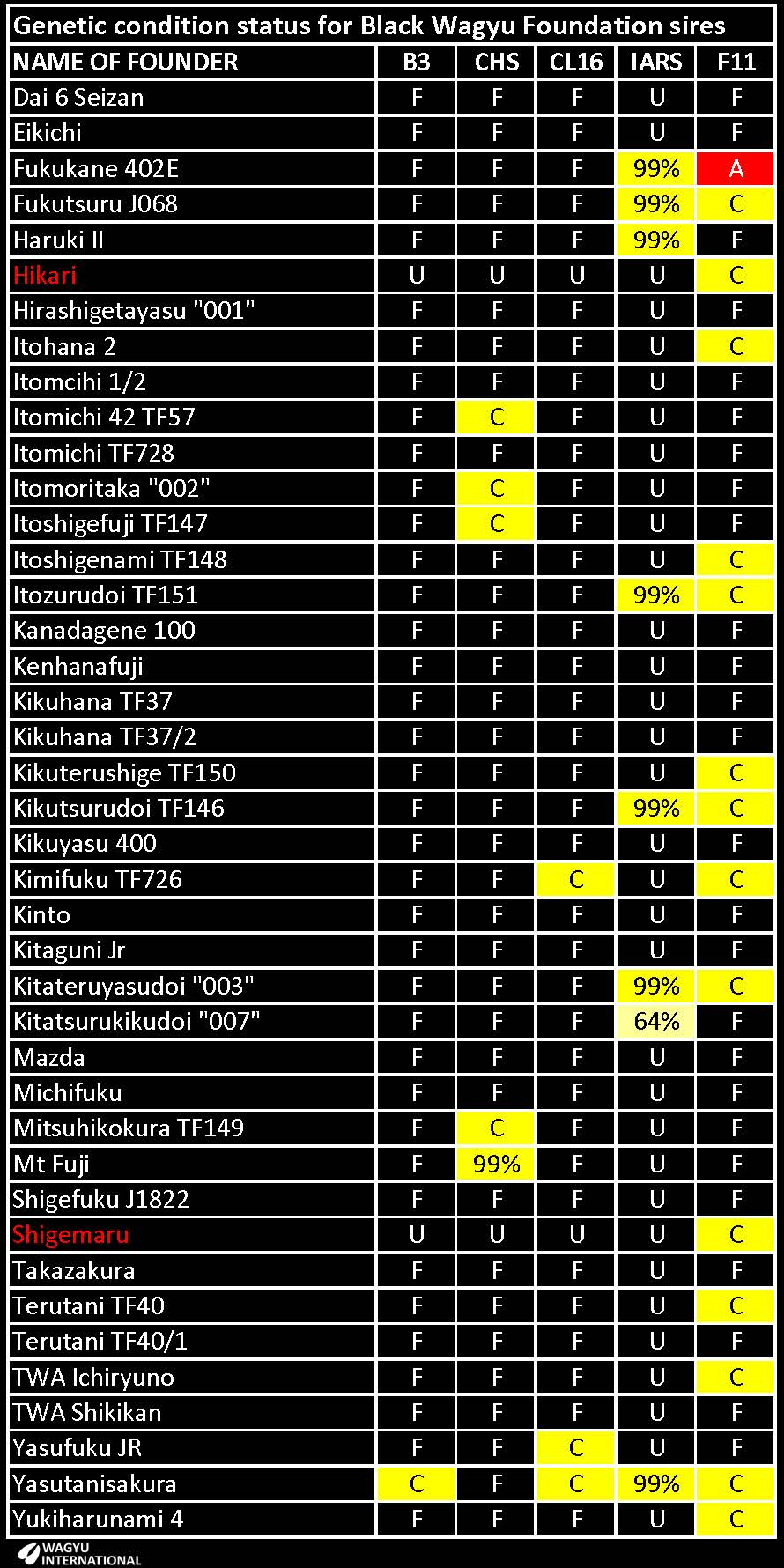
A - Determined to be homozygous Affected with two mutant genes from testing.
C - Determined to be heterozygote Carrier with one mutant gene from testing.
F - Tested to be free.
U - Untested, but based on pedigree is expected to be free.
% - The chance that an animal may be a carrier. Untested, but based on pedigree. The higher the percentage, the higher the probability that the animal is a carrier. 99% probability is the maximum.
The probability that the following Foundations have recessive conditions is tabled:
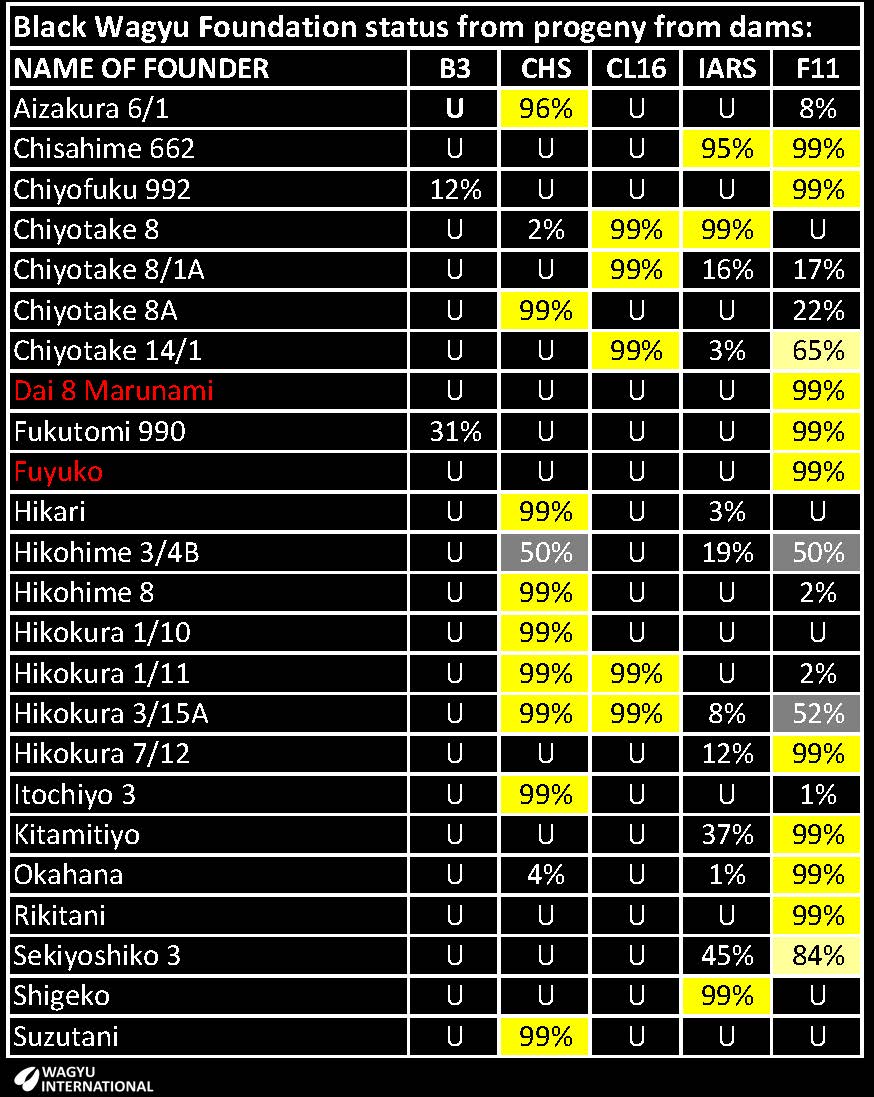
Control of hereditary conditions
A few high performers are carriers of hereditary conditions and the heavy use of artificial insemination and line breeding has resulted in the spread of some of these conditions from foundation animals. Carriers were exported from Japan and then on to other countries before their recessive genetic status was known. A table showing results from tests of Foundation animals up to 26th February 2011 is on the American Wagyu Association website.
Now that these conditions have been recognised and carriers and affected animals have been identified, related animals that may be at risk can be tested and identified too. It is becoming standard practice for all breeding animals to be tested before they or their genetics is offered for sale or export.
GeneProb software applies the results from tests across the pedigree database and the probabilities that animals are carrying any of the four genetic conditions is available. At-risk animals can be identified and decisions can be made to see if any further testing is required. When an animal has a probability of 50% or higher for one condition, it has at least one parent that has been tested to be a carrier of that condition, or else one of their progeny has been found to be a carrier. Previously this information was only available on a herd by herd basis.
Carriers with superior breeding can be retained but they should not be mated to other carriers. Their progeny that are tested to be free can be safely retained for breeding or sale.
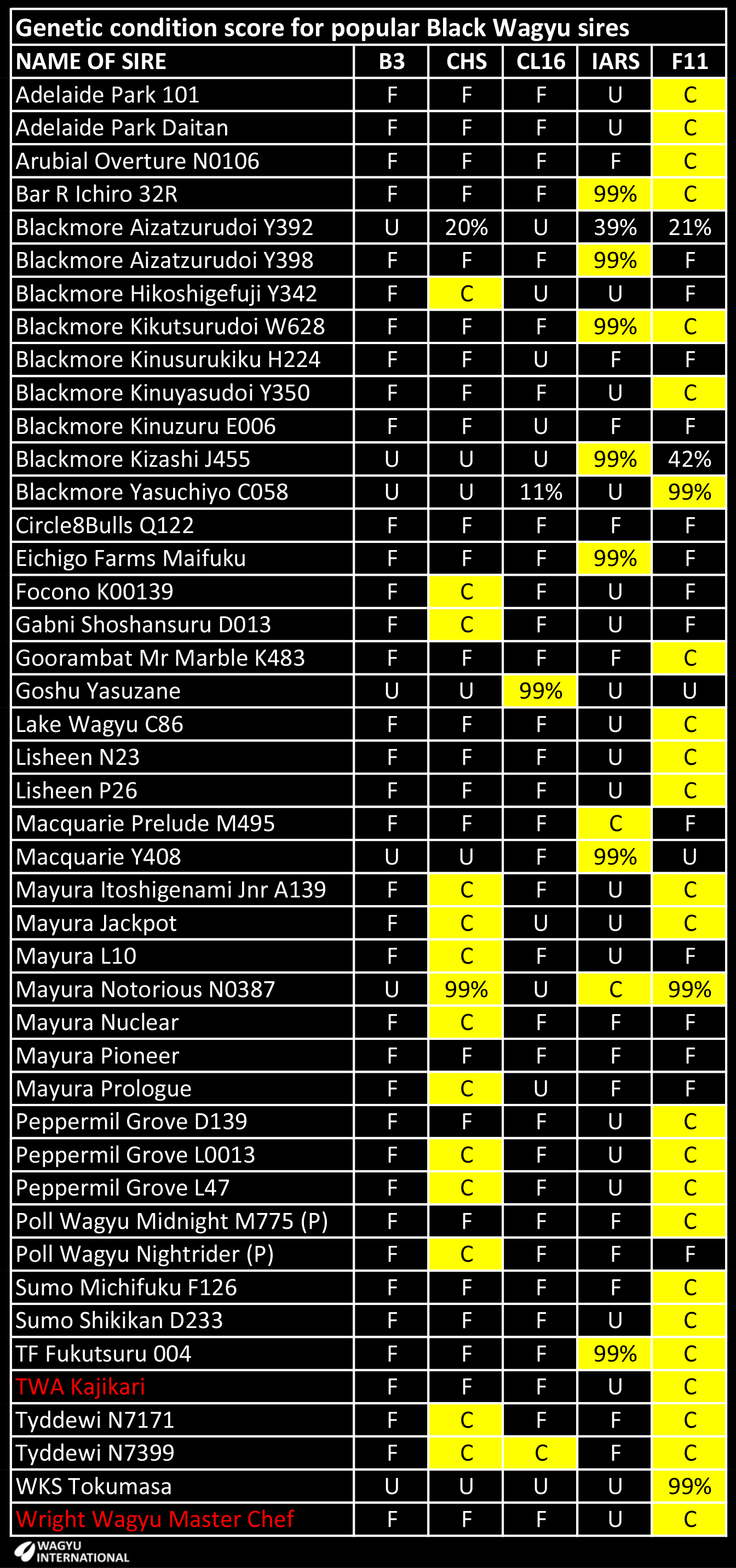
Summary of Recessive conditions in Export and popular animals from ABRI GeneProb
Test results are displayed in the American and Australian Wagyu Association webpages. This table shows test results and probabilities for Wagyu animals that are advertised in the public domain for export. Contact the owner for the current status as not all test results have been declared.
Sires:
Donors:
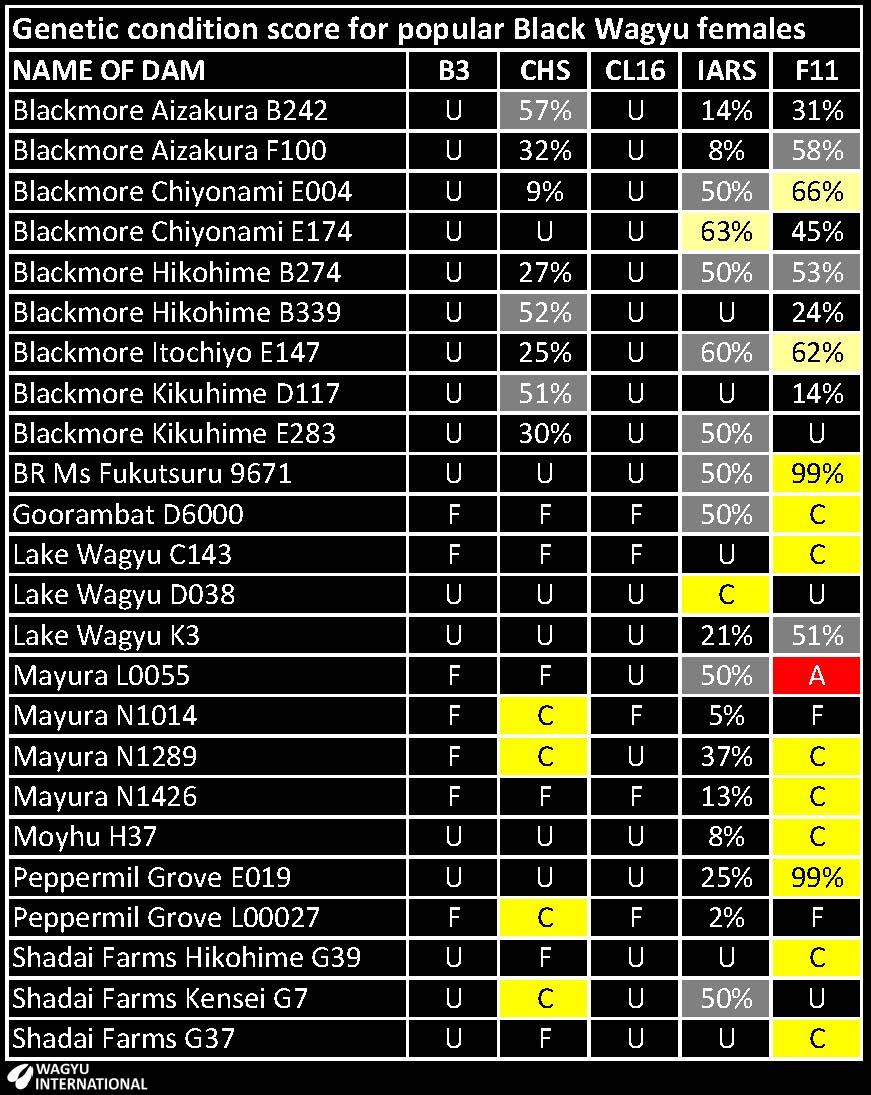
B3 - Spherocytosis. Lethal recessive genetic condition causes bleeding but growth can be retarded.
CHS - Chediak-Higashi syndrome. Immunity and clotting affected. Lethal.
CL16 - Renal tubular dysplesia / Claudin 16 deficiency. Kidney failure. Lethal.
IARS - Isoleucyl‐tRNA synthetase syndrome. Low birth weight, ill-thrift and embryonic death.
F11 - Factor XI deficiency. Prolonged bleeding time but not lethal.
C - Determined to be heterozygote Carrier from testing.
F - Tested to be free.
U - Untested but based on pedigree is expected to be free.
% - The chance that an animal may be a carrier. Untested, but based on pedigree. The higher the percentage, the higher the probability that the animal is a carrier. 50% indicates that one parent is likely to be a carrier. Contact the breeder for unpublished results when a percentage is displayed from GeneProb - especially when it is 50% or higher.
Please inform the webmaster if more recent test results have been obtained than these that are displayed.
References
American Wagyu Association animal database.
American Wagyu Association/iGenix. 2011. Inherited Genetics Disorder test results for AI Sires tested as of 26-2-2011.
Australia Wagyu Association animal database.
Australian Wagyu Association. 2012. Managing recessive genetic conditions in Wagyu in Australia. Australian Wagyu Update, Edition 51:3.
Australian Wagyu Association. 2020. Breeding: IARS disorder testing now available.
Ghanem, M.E., Nishibori, M., Nakao, T., Nakatani, K. and M. Akita. 2005. Factor XI mutation in a Holstein cow with repeat breeding in Japan. J. Vet. Med. Sci 67(7):713-715.
Hirano, Takashi , Naohiko Kobayashi, Tamako Matsuhashi, Daisaku Watanabe, Toshio Watanabe, Akiko Takasuga, Mayumi Sugimoto and Yoshikazu Sugimoto, 2013. Mapping and Exome Sequencing Identifies a Mutation in the IARS Gene as the Cause of Hereditary Perinatal Weak Calf Syndrome. PLOS One, 5 (5):1-8
Hirano, T., Hirotsune, S., Sasaki, S., Kikuchi, T. and Y. Sugimoto. 2002. A new deletion mutation in bovine Claudin-16 (CL-16) deficiency and diagnosis. Animal Genetics 33:118-122.
Hirano, Takashi, Tamako Matsuhashi, Kenji Takeda, Hiromi Hara, Naohiko Kobayashi, Kazuo Kita, Yoshikazu Sugimoto and Kei Hanzawa, 2016. IARS mutation causes prenatal death in Japanese Black cattle. Journal Animal Biotechnology 28 (4):242-7
Hirano, Takashi, Shota Nishimura, Hiromi Hara, Yoshikazu Sugimoto & Kei Hanzawa, 2017. Mapping of Calf Death in Japanese Black Cattle. Journal Animal Biotechnology 28 (4):242-7
Inaba, M., Yawata, A., Koshino, I., Sato, K., Takeuchi, M., Takakuwa, Y., Manno, S., Yawata, Y., Kanzaki, A., Sakai, J-i., Ban,A., Ono, K-i. and Y. Maede. 1996. Defective anion transport and marked Spherocytosis with membrane instability caused by hereditary total deficiency of red cell Band 3 in cattle due to a nonsense mutation. J. Clin. Invest. 97(8):1804-1817.
Kunieda, T. 2005. Identification of genes responsible for hereditary diseases in Japanese beef cattle. J. Anim. Sci. 76:525-533.
Ogata, Y, T Nakao, K Takahashi, H Abe, T Misawa, Y Urushiyama and J Sakai 1999. Intrauterine growth retardation as a cause of perinatal mortality in Japanese black beef calves. Zentralbl Veterinarmed A. 1999. 46(6):327-34.
Ohba, Y., Takasi, M., Nishii, N., Takeda, E., Maeda, S., Kunieda, T. and Kitagawa, H. 2008. 2007. Pedigree analysis of Factor XI deficiency in Japanese Black cattle. J. Vet. Med. Sci. 70(3):297-299.
DISCLAIMER Wagyu International provides information that has been supplied by other parties and gives no warranty (express or implied) as to the data completeness, accuracy or fitness for a particular purpose.